Compounds to crystallize
Potassium Aluminium Sulfate
Also known as "Alum" or "Potassium Alum" and has the chemical formula of KAl(SO4)2.12H2O, gives transparent octahedral crystals. Solubility: 118 g/L (20°C, water).
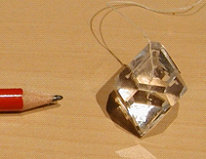
Credit: picture by Luc Van Meervelt
Disodium Tetraborate
Also known as "Borax" and has the chemical formula of Na2B4O7.10H2O, gives transparent crystals. Solubility: 27 g/L (anhydrous, 20°C, water).

Credit: picture by Luc Van Meervelt
Copper(II) Sulfate Pentahydrate
Has the chemical formula of CuSO4.5H2O, gives blue crystals. Solubility: 390 g/L (anhydrous, 20°C, water).

Credit: picture by Luc Van Meervelt
Monopotassium phosphate
Also know as "KDP", has the chemical formula of KH2PO4, gives transparent long crystals. Solubility: 22 g/100 mL (anhydrous, 25°C, water).

Credit: picture by Luc Van Meervelt
Ammonium Magnesium Sulfate Hexahydrate
The crystallization is started from ammonium sulfate and magnesium sulfate. Dissolve at room temperature equivalent amounts of both salts in water (e.g. 0.4 mol of both compounds in 45-48 mL water). Stir until everything is dissolved. Now make the solution supersaturated by adding small amounts (2 to 5 gram) of both salts under gentle heating (max. 30-40 °C). Cover the beaker and allow to cool at room temperature. Now proceed with the crystal growth in the usual way.

Credit: picture by Luc Van Meervelt
Potassium Sodium Tartrate
Also know as "Seignette salt" or "Rochelle salt", has the chemical formula of KNaC4H4O6.4H2O, gives transparent long crystals. Solubility: 630 g/L (anhydrous, 20°C, water).

Credit: picture by Luc Van Meervelt
Ammonium Iron(II) Sulfate Hexahydrate
Also know as "Mohr's salt", has the chemical formula of (NH4)2Fe(SO4)2.6H2O, gives light green crystals. Solubility: 269 g/L (20°C, water).

Credit: picture by Luc Van Meervelt
Potassium Ferricyanide
Also known as "Red Prussiate of Potash" and has the chemical formula of K3Fe(CN)6. The recipe below gives red monoclinic crystals. Solubility: 464 g/L (20°C, water).
Dissolve 93 grams of potassium ferricyanide in 200 cc of warm water, cover the solution, and allow it to cool.

Credit: picture by Wayne Schmidt (http://www.waynethisandthat.com/crystals.htm)
Copper Acetate Monohydrate
Chemical formula: Cu(CH3COO)2.H2O. The recipe below gives blue-green monoclinic crystals. Solubility: 72 g/L (20°C, water).
Dissolve 20 grams of copper acetate monohydrate in 200 cc of hot water. If a scum of undissolved material persists, add a few drops of acetic acid and stir well. Cover this solution, and allow it to cool and stand for a few days; usually it will deposit crystals spontaneously.

Credit: picture by Coba Poncho
Calcium Copper Acetate Hexahydrate
Chemical Formula: CaCu(CH3COO)2.6H2O. The recipe below gives blue, tetragonal crystals.
Add 22.5 grams of powdered calcium oxide to 200 mL of water, pour into the mixture 48 grams of glacial acetic acid, and stir until the solution is clear. If there is a small insoluble residue, filter the solution. Dissolve separately 20 grams of copper acetate monohydrate in 150 cc of hot water. Mix the two solutions, cover the mixture, allow it to cool for a day. If it does not deposit crystals spontaneously, let a drop of the solution evaporate and scrape the resulting seeds into the bulk of the solution.
Sodium Chloride
Chemical Formula: NaCl. Also known as table salt. Solubility: 35.9 g/100 mL (20°C, water).
NaCl tends to form smaller crystals, or less well formed crystals because the solubility barely changes at all as a function of temperature; at 20°C, one can dissolve 35.9g of NaCl in 100g of water, and at 100°C, just 39.2g per 100g of water. The most appropriate method of growing sodium chloride crystals is therefore by evaporation of a saturated solution. Small (sub-millimetre) clear cubes with smooth faces will grow on the bottom of your glass dish or jar, or on any thread suspended in the jar. Larger crystals tend to develop hopper faces, or even more erratic growth habits. One interesting experiment to try is to see how the growth morphology changes if you add small quantities of other substances - a smidge of copper sulfate perhaps, K-alum, or sodium nitrate - to your saturated salt solution.

Sucrose
Chemical Formula: C12H22O11. Also known as saccharose or table sugar. Solubility: 211.5 g/100 mL (20°C, water).
Cane sugar produces great crystals without too much trouble, provided you can be patient. Dissolve ~500 grams of sugar per 100 mL of hot water, and leave to cool. The pale silvery-yellow solution is very viscous when supersaturated, and can take from a week to over a month to start producing crystals, depending on how big a jar you're using. Crystals shoot from smaller volumes of liquid quite quickly and can grow to a length of a few millimeters. Use these as seeds to grow much larger crystals. You can grow very pretty single crystals over a period of just a few weeks. Sucrose is slightly deliquescent; in other words, the crystals 'sweat' a bit. You'll find the crystals become slightly moist and sticky, even when you've dried them, particularly in a warm room.
Fructose
Chemical Formula: C6H12O6. Also known as fruit sugar. Solubility: 3750 g/L (20°C, water).
Solutions are made up in the same way as for cane sugar. As with sucrose, patience is everything. An 80 wt. % fructose solution, if unseeded, may take several weeks to start crystallising at room temperature; the first sign is the appearance of little flat squares floating on the surface of the solution. If you instead seed the solution with some of the tiny crystals from your original box, then what happens is that white 'blotches' - like cotton wool - appear in the liquid. These might be mistaken for bits of mould; in fact they are aggregates of very very fine hair-like crystals of fructose hemihydrate (C6H12O6 • ½H2O). The hemihydrate will also appear if you seed a concentrated solution kept in the fridge (at 1 - 4°C). Vigorous stirring of the solution breaks up the hemihydrate crystals and causes crystals of fructose dihydrate (C6H12O6 • 2H2O) to nucleate.
Potassium chromium sulfate
Chemical formula: KCr(SO4)2•12(H2O). Also known as chromium alum. Gives deep purple crystals. You can grow a clear alum layer around the purple crystals.
- The growing solution will consist of a chromium alum solution mixed with an ordinary alum solution. Make a chromium alum solution by mixing 60 g of potassium chromium sulfate in 100 ml water.
- In a separate container, prepare a saturated solution of ordinary alum by stirring alum into warm water until it will no longer dissolve.
- Mix the two solutions in any proportion that you like. The more deeply colored solutions will produce darker crystals, but it will also be harder to monitor crystal growth.
- Grow a seed crystal using this solution, then tie it to a string and suspend the crystal in the remaining mixture.
- Loosely cover the container with a coffee filter or paper towel. At room temperature (~25°C), the crystal can be grown via slow evaporation for as little time as a few days or as long as a few months.
- To grow a clear crystal over a colored core of this or any other colored alum, simply remove the crystal from the growing solution, allow it to dry, and then re-immerse it in a saturated solution of ordinary alum. Continue growth for as long as desired.




