Molecular structures
Posted on 24/01/2014

David Turner
You must be taking the **** – Urea
What does it look like?
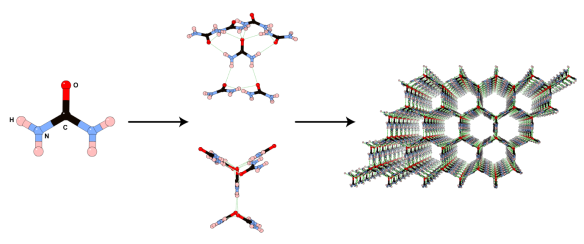
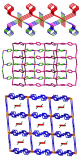
Image generated with Crystal Maker software http://www.crystalmaker.com/
What is it?
Urea is a simple, biologically important molecule which was first isolated from urine in the early 18th century. Urea provided a massive turning point in the chemical sciences, being the first biologically known chemical that was synthesised in a laboratory proving that 'living matter' and 'non-living matter' are made of the same building blocks and disproving the generally held theories of the time. Urea is formed in the body of mammals during the metabolism process of nitrogen-containing compounds and is then passed in our 'liquid waste'. Due to the high percentage nitrogen content of urea, and extremely low toxicity, it is used as a nitrogen-rich plant fertilizer, which can also be applied direct from source according to some . . .
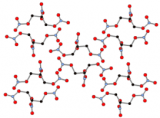
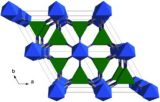
Whilst urea has several crystalline forms the one represented above is, from a personal perspective, the most fascinating. In the presence of suitable co-crystallising molecules, urea can form infinite hexagonal channels due to its ability to form many hydrogen bonds. Long alkane derivatives are used as the co-crystallising agents to act as templates around which the channels are able to form, giving a clathrate material – where guest molecules are incorporated into the crystal lattice of another. These types of material are not dissimilar to clathrates of water (see Jan 18th post).
Where did the structure come from?
Whilst many examples of urea clathrates have been reported, this particular structure was reported in 1997 by Yeo and Harris and has 1,10-dibromodecane as a guest species in the channels.
Related articles
|
Interdigitation, Interpenetration, Intercalation |
A Nobel Explosive – Trinitroglycerin |
Shrinking in the heat – lanthanoid hexacyanidocobaltates, a.k.a. LnCo(CN)6 |