Molecular structures
Posted on 18/01/2014

Helen Maynard-Casely
Fire ice – the structure of methane hydrate I
What does it look like?
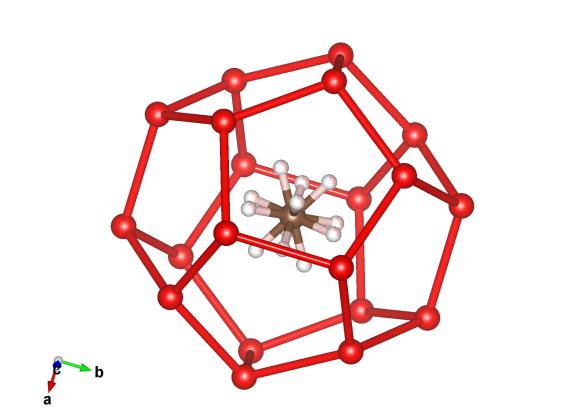
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
One of the cages in the methane hydrate I crystal structure. The red atoms in this structure are the oxygen postions in the water molecules (their hydrogens have been removed to make things a bit clearer). The brown and pink atoms represent the carbon and hydrogen in the methane molecule which sits inside the cage and spins. Hence why there is more than four hydrogen atoms drawn in this molecule.
What is it?
Though it will look like normal ice, and be pretty cold to the touch, you don’t want to get a lighted match near this material. Methane hydrate, or 'Fire Ice', forms when you pressurise methane and water and cool it down. The water forms a cage which traps the methane molecules, this is known as a clathrate structure.
This is a host-guest structure, with the water 'host' molecules containing the methane 'guest' molecules. To contain methane with water in this, delicate, structure requires temperatures to be below 25°C and the molecules to be buried by about 300m of sediment or ice. Humphry Davy (a rather interesting character who wrote romantic poetry aside from being a leading chemist) discovered clathrate materials in 1810, when he pressurised water with chlorine gas. Since then we have discovered that these structures form in a number of gases: Nitrogen, Argon even Hydrogen.
There are also a number of ways that the water forms a cage about the gas molecule it's hosting. These differ in the size and variety of cages that they contain. The research into these has opened up a number of intriguing possibilities of how we can engineer and store a lot of gas in a small amount of space.
Where did the structure come from?
This is structure #4112809 in the Crystallography Open Database.
Related articles
|
A structure determined by Linus Pauling – Hematite |
A material for another world – Sulfuric Acid Hexahydrate |
Biology on the edge – Adaptor Protein complex 2 |