Molecular structures
Posted on 12/01/2014

Helen Maynard-Casely
Sweet crystallography – the crystal structure of sucrose
What does it look like?
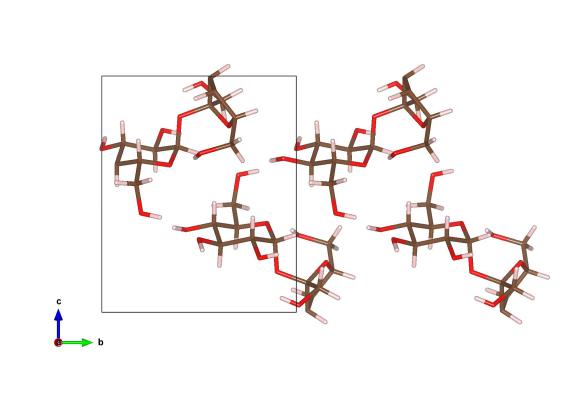
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
What's within that sugar cube floating in your coffee? There are a number of forms of sugars, but the one that sits as a white powder in our cupboards is sucrose. The sucrose molecule itself is a combination of a glucose and fructose molecule (you can see that each of the molecules has two parts). This means it has a very specific way of packing together to form a solid. Each sucrose molecule is made of carbon (brown), oxygen (red) and hydrogen (pink).
Where did the structure come from?
Given how important sucrose is to our diets, and how much money is made from sugar, finding its crystal structure was very important question of early crystallography. One of the challenges to finding its structure was the fact that it is composed entirely of 'light elements' - only carbon, oxygen and hydrogen. The first structure was published in 1952 by Beevers et al. after they collected the data in 1944 (from a crystal provided by UK syrup manufacturers Tate and Lyle). But this structure only proposed the locations of the carbon and oxygen atoms – it wasn't until 1963 that Brown and Levy found where the hydrogen positions were.
This is crystal structure number 3500015 in the open crystallography database.
Related articles
|
A long chain crystal – Silver behenate |
Absorbing gas – Lithium isonicotinate.solvate |
You must be taking the **** – Urea |