Molecular structures
Posted on 04/01/2014

Helen Maynard-Casely
A material for another world – Sulfuric Acid Hexahydrate
What does it look like?
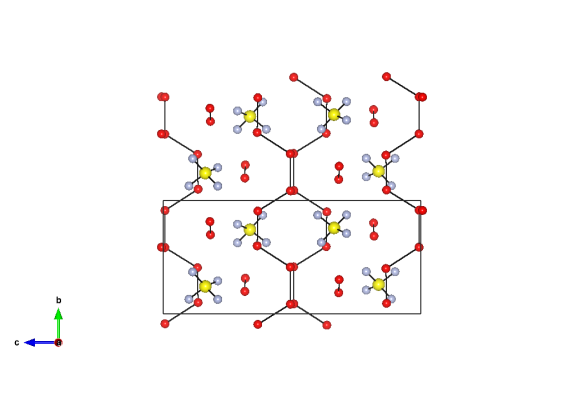
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
This material forms when a water-rich mixture of sulfuric acid and water is cooled below 200 K (about -80 °C). It is a mix of six water molecules to one sulfate molecule. Most of the water molecules (which are represented by the red atoms) form in layers with the ones left over filling the gap with the sulfate molecules (blue and yellow atoms). The box that can be used to describe the structure is 7.4 x 7.5 x 17.0 Å. Though it probably doesn't form all that much on Earth, it could occur on the surfaces of the icy moons of Jupiter – Europa, Ganymede and Callisto. The sulfur that could make this material is thought to be transported from the volcanoes of their sister moon Io, via the magnetic field of Jupiter, before being bombarded onto the icy surfaces.
Where did the structure come from?
This structure was determined from synchrotron X-ray diffraction, and was only discovered last year. It's reported in Maynard-Casely et al. 2013.
Related articles
|
Botox: Toxic medicine |
An Earth and Mars mineral – Meridianiite MgSO4.11H2O |
20141231_original |