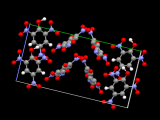
Molecular structures
Posted on 06/03/2014

Helen Maynard-Casely
The sour in the sweet – citric acid
What does it look like?
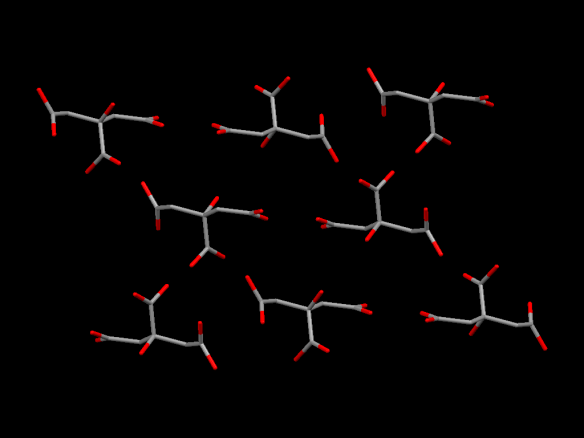
Image generated by the Mercury crystal structure visualisation software http://www.ccdc.cam.ac.uk/Solutions/CSDSystem/Pages/Mercury.aspx
What is it?
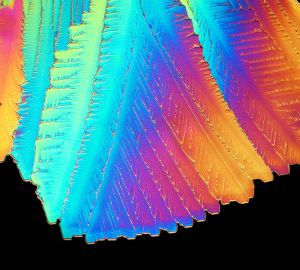
Crystals of citric acid in polarised light by Jan Homann
Yes, at Crystallography365 towers pancake day came and went and we didn’t notice (or have any pancakes). But it's probably time we blogged about another foodstuff and today it's the turn of citric acid. Normally a white powder, citric acid can form as an anhydrous (without water) form, which is what we've pictured, and a monohydrate (along with one water atom). It can be grow into lovely crystals that look beautiful under polarised light.
Most often used as a sour taste, citric acid was first isolated from Lemon juice in 1784. It's often used as a food additive, but is also a vital ingredient in sherbet.
Where did the structure come from?
We got the co-ordinates for this the arrangement of citric acid molecules from a paper by Nordman, Weldon and Patterson published in 1960.
Related articles
|
The chocolate you don’t want – cocoa butter form VI |
Less than mellow yellow – the structure of picric acid |
An extra-terrestrial hydrocarbon – Ethane |