Molecular structures
Posted on 27/02/2014

Joseph Bevitt
Plastic fantastic – polyethylene
What does it look like?
![Crystalline structure of polyethylene. (a) Side-view of orthorhombic structure of polyethylene. (b) Projection of unit cell on the ab plane The larger circles represent C and the smaller circles represent H.[1]](https://www.iycr2014.org/__data/assets/image/0003/106428/polyethelene2.jpg)
Crystalline structure of polyethylene. (a) Side-view of orthorhombic structure of polyethylene. (b) Projection of unit cell on the ab plane The larger circles represent C and the smaller circles represent H.[1]
Polyethylene, the most commonly used plastic and synthetic polymer, is a recyclable thermoplastic that can be melted and blown, poured or pressed into a multitude of useful shapes. Polyethene comes in many structural forms classified by density, degree of chain branching and crystallinity. The two most well-known variants are low-density polyethylene (LDPE) and high-density polyethylene (HDPE).
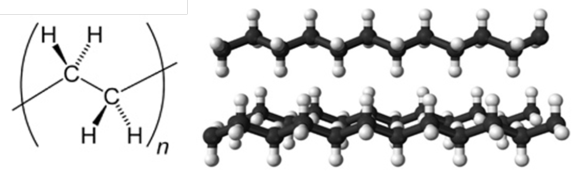
The repeating unit and chains of polyethylene.
LDPE is composed of highly-branched polymer chains that do not pack closely together and are randomly oriented relative to one another, i.e. LDPE is amorphous. The large distance between adjacent polymers weakens the intermolecular forces between polymers which are easily overcome, thus LDPE has a low melting point and is highly flexible. LDPE is hence used to make cling wrap, food containers and playground slides. LDPE was first produced in 1933 by the free radical polymerisation process. The recycling number for LDPE is 4.
HDPE polymers have a low degree of branching that pack closely together in highly ordered or crystalline arrays. HDPE has a greater density than LDPE and stronger intermolecular forces, thus is harder, more rigid and has a higher melting point. HDPE is used to make water bottles, water pipes, hard hats and garden furniture. The recycling number for HDPE is 2.
Where did the structure come from?
Early X-ray diffraction measurements by C.W. Bunn and co-workers at ICI determined that polyethylene formed materials that were about 50% amorphous and 50% crystalline. This property, a mixture of order and disorder, makes polyethylene and other polymers very difficult (but also very interesting) to study.
[1] Density Functional Analysis of Impurities in Polyethylene http://electronicstructure.wikidot.com/density-functional-analysis-of-impurities-in-dielectrics. Accessed 24 Feb 2014.
[2] Structural Characterization of Blown Films of High Density Polyethylene and its Relationship to Moisture Vapor Transmission Rate http://www.che.vt.edu/Faculty/Wilkes/GLW/daves_page/daveweb.htm. Accessed 24 Feb 2014
Related articles
|
Less than mellow yellow – the structure of picric acid |
Getting in a Twist – A Polyrotaxane |
Hero and a villain – Jarosite |