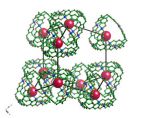
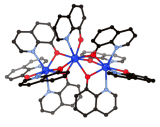
Crystals by Design – Copper Tetracyanotetraphenylmethane Tetrafluoroborate
What does it look like?
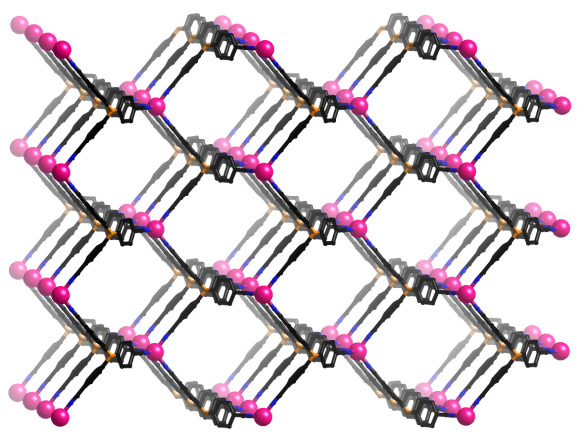
Image made using the Diamond Visualisation software.
What is it?
The properties of materials, covering such diverse things as whether they dissolve, whether they conduct electricity, whether they flex or crack under pressure, or even their colour, are very dependent on how the molecules are arranged within them. It is therefore vital, if one is trying to design new materials, to be able to control the way the molecules are arranged. This, until recently, has been extremely difficult. When a solid forms, such as when (as Carl Sagan might say) billions and billions of molecules come together to form a crystal, the molecules usually make up their own minds about how they want to arrange themselves, and they do so in ways that are often difficult to predict or control.
This material shown above is therefore an incredibly important landmark. Reported in 1989 by Richard Robson and Bernard Hoskins at the University of Melbourne, it was the first coordination compound in which the constituent organic ligands and metals come together to form an infinite network with a deliberately designed arrangement. Furthermore, the design leads directly to an unusual property – the framework only makes up about 1/3 of the volume of the crystal, with the rest essentially a soup of anions and solvent.
The design approach was remarkably simple and elegant. Take a well known inorganic compound and essentially copy the way the atoms are connected, but with metals and large organic molecules rather than single atoms. In this case, the structure is based on diamond, which is composed of carbon atoms with tetrahedral geometries connected together by carbon-carbon bonds. Instead of using carbon atoms, however, this compound was made by mixing large tetrahedral molecules with copper atoms, which also like to have a tetrahedral arrangement of molecules around them. Mix these two compounds together in the right solvent and hey! presto! they spontaneously arrange themselves into an infinite diamond-like network, exactly as predicted! This time, however, the tetrahedral centres are not separated by 1.54 Å (the length of the carbon-carbon bond in diamond), but 8.86 Å, nearly a sixfold increase. This creates huge pores through the structure that are not present in diamond itself.
Today these materials (coordination polymers, also often called metal-organic frameworks, or MOFs) are extensively studied by hundreds of research groups around the world, usually using the same design procedure pioneered with this compound. Applications include capture and storage of gases such as hydrogen, carbon dioxide or methane (important, for example, for hydrogen-fuelled transportation (where water is the only waste product) and capture of greenhouse gases), catalysis (making chemical reactions go faster and more cleanly), separations of different chemicals, molecular sensing (e.g. pollutants and toxins), and materials with unusual magnetic, electronic, optical or other properties. Hundreds of applications, tens of thousands of new compounds, but all descendants, in one way or another, of this remarkable example of the design of solids on the molecular level.
Where did the structure come from?
This structure was first reported in: "Infinite Polymeric Frameworks Consisting of Three Dimensionally Linked Rod-like Segments", B.F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962. It is deposited in the CCDC Refcode: JARMEU