Triglycine Sulphate: Ferroelectic, Pyroelectric, Bolometric
What Does it Look Like?
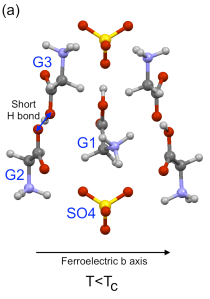
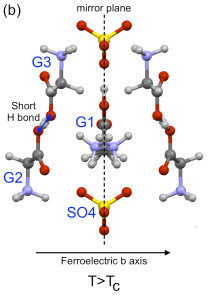
Figure 1: (a) Shows part of the unit cell of TGS in the ferroelectric phase (T < TC) and (b) shows the paraelectric phase (T > TC). In the paraelectric (non-electrically polarised) state, the middle glycine, G1, is just as likely to be 'bent' to the left as to the right, but below TC the molecules 'choose' a side and a net polarisation results.
What is it?
Triglycine sulphate, (TGS), (NH2CH2COOH)3H2SO4 is a molecular ferroelectric. It is used as a detector in IR spectroscopy, amongst other things.
In a ferroelectric crystal (for example PZT), each unit cell has a small electric polarisation — roughly speaking, the 'centre of mass' of the positive charge distribution is different from that of the negative charge. If all the cells in a crystal have polarisations pointing the same way, the crystal will be electrically polarised. This is useful in a number of ways — for example, piezoelectric materials (like quartz and newer materials like PZT) can be used as little motors and actuators, and for turning waste motion into electricity.
In TGS, the electrical polarisation comes mostly from the NH3+ group on the G1 molecule, which is disordered at high temperature (well, the critical temperature TC is only 49°C in TGS and 62°C if you replace the H with deuterium, so not very high temperature). This means the molecule — G1 in Figure 1 — looks symmetrical and in fact the left half is the mirror of the right (and vice versa). As it is cooled down the NH3+ group ceases to be equally likely to be to the left or right, and settles down on one side of the mirror — breaking the symmetry. Through a combination of molecular interactions and electrostatics, nearby molecules choose the same side, and this spreads to the whole crystal (especially if it is cooled in an electric field), giving an overall polarisation.
This does not all happen at once. By looking at diffuse scattering, that is the X-ray (or neutron) diffraction between the strong sharp Bragg peaks, we can get an idea of the short-range order. We can see when the NH3+ groups are only correlated (pointing the same way) for a few unit cells at a time. In such a state, the average unit cell still shows 'random' NH3+ orientations, but the real structure is not quite like that …
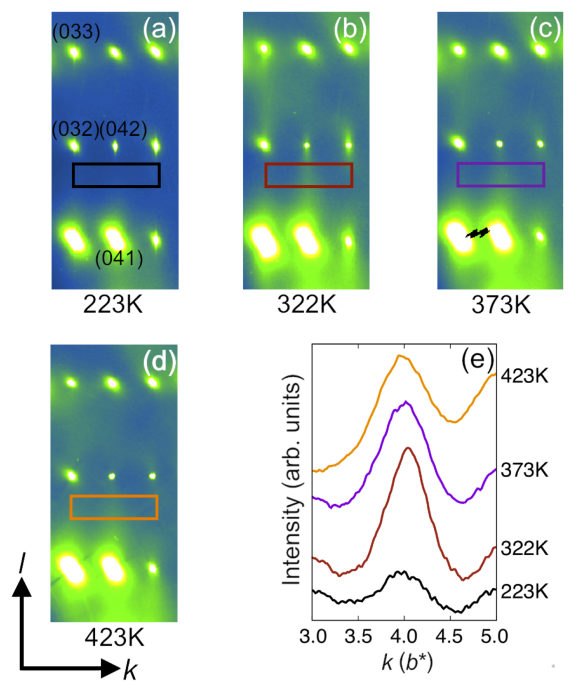
Figure 2: Maps of a small region of reciprocal space (the diffraction pattern) measured at different temperatures. The rectangles enclose a streak of scattering that comes from small regions in the TGS crystal where NH3+ groups are all lined up the same way. (e) Shows that this streak is most intense at 322K, which is the critical temperature. Above this temperature, the small regions shrink and below it the long-range order sets in, also causing the small domains to vanish.
Where did the structure come from?
The structure of TGS has been known for a long time. The work on its short-range order is more recent.