Mineral structures
Posted on 22/01/2014

William Brant
The first perovskite – Calcium Titanate
What does it look like?
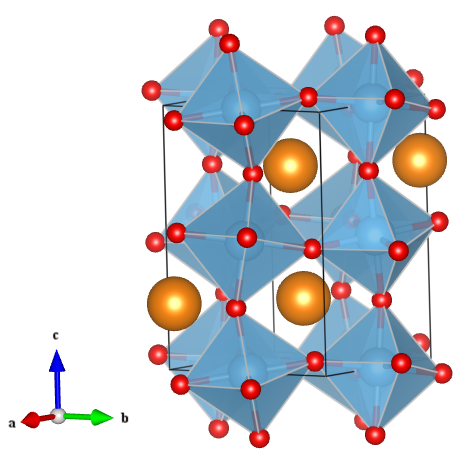
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
One of the most common and versatile structure types, perovskites, cannot be considered without first paying homage to the original perovskite, calcium titanate. First discovered in the Ural Mountains of Russia by Gustav Rose in 1839, the mineral was named after the mineralogist Count Lev Aleksevich von Perovski.
Starting from a face centered array of anions, the perovskite structure can be derived by replacing one pair of adjacent anions (labelled as X) in the unit cell by a similarly sized cation, referred to as the A-site cation. Smaller B-site cations can then be placed in interstitial sites such that they are surrounded by six anions. This results in the formula ABX3, which is the generic formula for a perovskite. A visual representation of this transition is shown below where the A-site cation is indicated in orange, the B-site in blue and the anions are red.
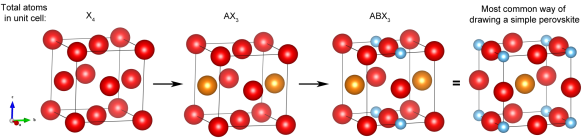
How to build a perovskite
Due to the high versatility of the perovskite structure, virtually every element in the Periodic Table can be incorporated into a perovskite structure, resulting in a vast array of properties and applications. For calcium titanate specifically, calcium is the large A-site cation, titanium the smaller B-site cation and oxygen the anion, giving the formula CaTiO3 (also shown in the figure).
CaTiO3 is arguably one of the less interesting perovskite structures, compared to its more exotic cousins. Nonetheless, one of CaTiO3's main uses is as one of the major components in Synroc, a synthetic rock form designed for the immobilization of radioactive waste, due to the ability to host for the nuclear fission product strontium.
Where did the structure come from?
While on-going advances in both crystal growth and diffraction techniques have enabled more accurate measurements of the CaTiO3 crystal structure, it largely has not changed from the distorted orthorhombic perovskite structure determined by Kay & Bailey in 1957.
Related articles
|
May's Birthstone: Could you actually build an Emerald city? |
Decorative, but a little deadly – Torbernite |
Seeing Double – Calcite |