Mineral structures
Posted on 18/03/2014

Helen Maynard-Casely
It's in the walls – Alite
What does it look like?
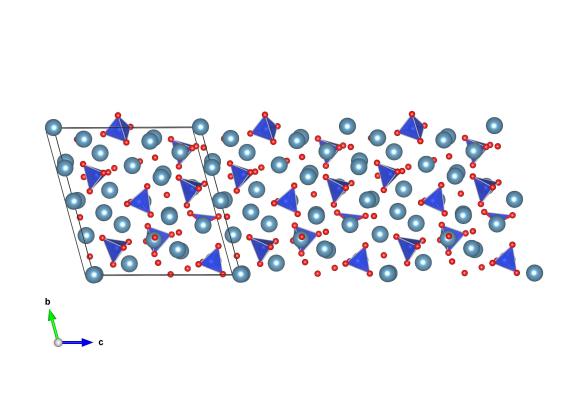
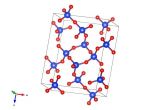
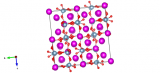
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
This material is everywhere: it's probably holding up where you're currently sitting and in the walls all around you. Alite, which is one name for tricalcium silicate, is one of the components of Portland cement and is responsible for turning this liquid rock into a strong solid. It has roughly three calcium atoms in the structure for every silicate, but often has a number of other materials incorporated into its crystal structure, such as magnesium, sodium and iron oxides. The amount of these can be tuned when mixing up the cement, and can alter the physical properties of the cement to suit your purpose. Be it quick drying or a slightly stronger setting, a small change in the materials in this crystal structure can make all the difference.
Where did the structure come from?
As a material which is a 'solid solution' or mixture of materials, it is often a struggle to pin down the exact composition of alite. However this work by De la Torre et al. controlled the composition of the alite they formed in the laboratory, and charted how this affects its crystal structure. The image we've made is from the alite formed with very little magnesium in it.
Related articles
|
Biominerals #4 – The wonderful world of silica |
Beautifully holding buildings together – Tricalcium aluminate |
Vinegar – acetic acid |