Mineral structures
Posted on 07/03/2014

Helen Maynard-Casely
Classical crystal structures – sphalerite
We at Crystallography365 towers are going on a short break this weekend – but don’t panic. We've scheduled up three short posts of 'classical' crystal structures that form from two elements (binary compounds). These are structures that often appear in the scientific literature, and are ones that most crystallographers (who are worth their sodium chloride) will know about! Indeed that was a clue to the fact that one has already been covered (the 'NaCl' structure). Today is the turn of the cubic form of ZnS, Sphalerite.
What does it look like?
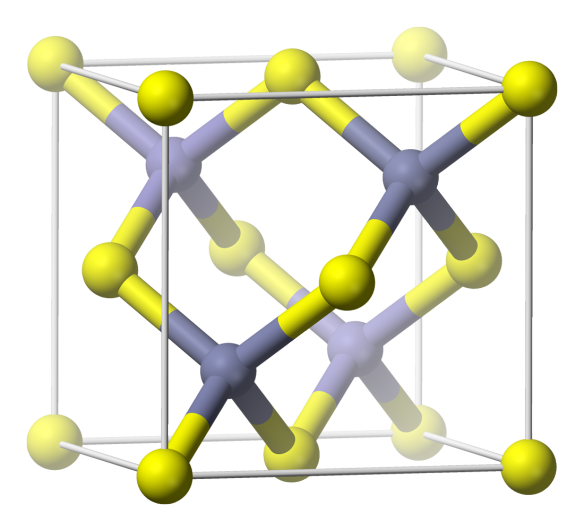
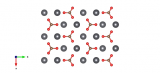
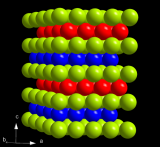
The cubic crystal structure of zincblende or sphalerite
What is it?
Sphalerite (often known as Zincblende) is made up of zinc and sulfur and is the cubic crystal structure formed from these two elements. This crystal structure, and its hexagonal equivalent Wurtzite, typically occur when elements with radii a bit smaller than NaCl form into solids. Each of the atoms in the structure bonds to four others, making for quite a tough arrangement. Many binary element mixtures take up this structure, including Boron Nitride and Silver Iodide.
Where did the structure come from?
The structure of zincblende was one of the first ever crystal structures to be determined, by W. L. and W. H. Bragg. A crystal structure of natural sphalerite is #1011232 in the Crystallography Open Database, but this has a substitution of about a third of the zinc atoms for iron atoms.
Related articles
|
Mineral in pink – Spherocobaltite |
Celebrating Laue – very tiny crystals |
Madam Curie – one of her elements: Curium |