Inorganic structures
Posted on 31/01/2014

Paige Hawkins
Spinning around with Spinels – Lithium titanate
What does it look like?
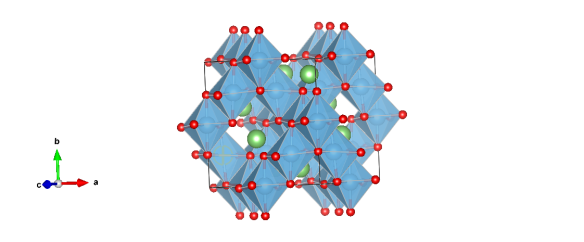
Image generated by the VESTA (Visualisation for Electronic and Structual analysis) software http://jp-minerals.org/vesta/en/
Octahedral sites are occupied randomly by lithium (1/6) (not shown in image) and titanium (5/6) atoms. Ti atoms are blue, Li atoms green and O atoms are red.
What is it?
Lithium titanate has a spinel-type structure and is a promising anode candidate for long-life lithium-ion batteries. Current lithium-ion batteries use carbons, such as graphite, but these can feature safety issues when the battery is charged or used rapidly. Li4Ti5O12 is much safer to use at these rapid rates and only features minor structural changes (e.g. expansion/contraction) during battery function. Therefore, it is said to have excellent cyclability andlong term stability at a high capacity.
What makes up a battery?
 To the right is a schematic of a coin cell as can be constructed with lithium titanate as an anode material.
To the right is a schematic of a coin cell as can be constructed with lithium titanate as an anode material.
A rechargeable battery features chemical reactions that are approximately reversible with charge and use. A lithium-ion battery is rechargeable and relies on the transfer of lithium from the cathode to the anode during charge using electrical current and the reverse during use where it produces electrical current. It is found in many consumer portable electronic devices such mobile phones, computing and entertainment devices.
The lithium-ion battery industry is estimated to be worth $USD11.70 billion in 2012 and is forecast to reach $USD33.11 billion by 2019.
Where did it come from?
Cystallographic information on lithium titanate can be obtained from #4000741 in the Crystallography Open Database.
Related articles
|
Neo-mag: The strongest permanent magnet of them all! |
Crystals you can grow at home – Nickel Sulphate Hexahydrate |
Protein crystals naturally occurring in a cellular membrane: Bacteriorhodopsin |