Inorganic structures
Posted on 25/01/2014

Sara Callori
Buckminsterfullerene a.k.a. Buckyballs a.k.a. C60
What does it look like?
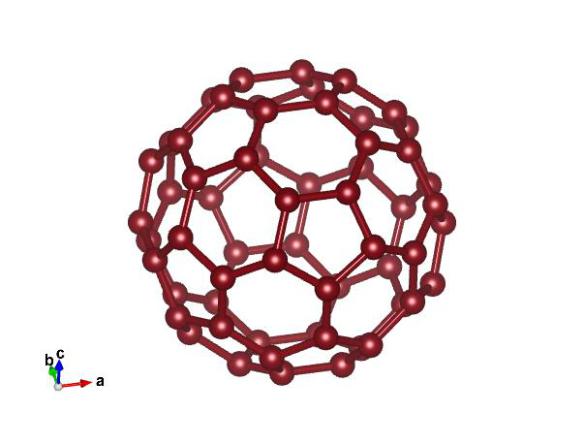
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
What looks like a soccer ball but has a lot more in common with a diamond? That would be buckminsterfullerene, the carbon structure shown above. With a chemical formula of C60 and a catchy nickname (the bucky-ball) this carbon structure is made up of twenty hexagons and twelve pentagons, which form a truncated icosahedron. Since buckyballs are empty in the middle, they can be used as "atomic shrink wrap", where a different atom is placed in the center of the C60 cage, which can then be "shrunk" by laser pulses to trap the atom. Like graphite, buckminsterfullerene is a sort mater, however, under pressure it transforms into a superhard structure which can even indent diamonds.
Where did the structure come from?
Buckyballs were first synthesized in the 1980's by Robert Curl, Harold Kroto, and Richard Smalley. The structure was named after architect Buckminster Fuller, who was well-known for designing geodesic domes. Their work was awarded the 1996 Nobel Prize in Chemistry. The structure is #9011073 on the Crystallography Open Database.
Related articles
|
Not going anywhere – low expansion alloys |
Madam Curie – one of her elements: Curium |
The crystal structure rainbow – Green fluorescent protein |