Inorganic structures
Posted on 23/01/2014

Mackenzie Hagan
WOOO, Tungsten(VI) Oxide!
What does it look like?
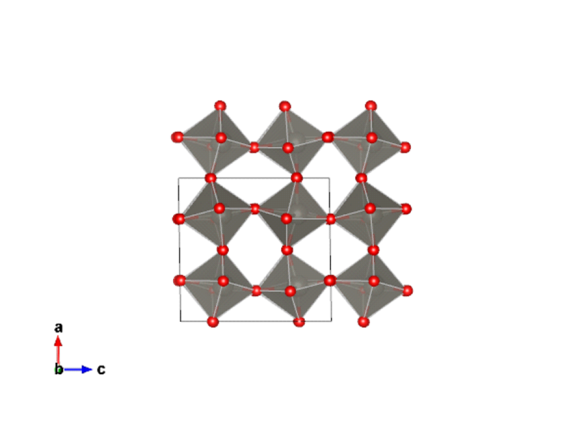
Image generated by the VESTA (Visualisation for Electronic and STructual Analysis) software http://jp-minerals.org/vesta/en/
Tungsten (VI) oxide's structure varies depending on temperature, the structure shown here is stable from 17-330°C. Red spheres are oxygen, spheres in the centre of each octahedron are tungsten.
What is it?
Tungsten (VI) oxide (chemical formula WO3), also known as tungsten trioxide, is an intermediate obtained from the recovery of tungsten from its minerals (e.g. tungstite or scheelite). Its uses are numerous and varied, ranging from the fireproofing of fabrics, to being used as a pigment in paint (due to its rich yellow colour). One of the most fascinating uses is in the production of "Smart Glass", a type of glass that changes its transmission properties when a voltage is applied to it, effectively going from transparent to translucent. Smart glass can be used in place of regular windows in cars, aircraft or buildings to control the amount of heat, light or glare allowed through the glass.
Where did the structure come from?
This structure is available in the Inorganic Crystal Structure Database (ICSD) #80056.
Related articles
|
SmB6: When interesting is skin deep |
Gd5Si2Ge2: Magneto gets cooler |
LixCoO2 – The Breakout Battery Hit |