Inorganic structures
Posted on 11/04/2014

Jonathan McCree-Grey
Murder most foul – Potassium Cyanide
What does it look like?
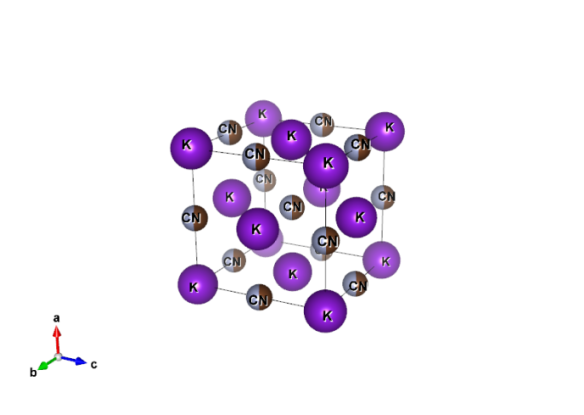
Image produced by Vesta crystal imaging software using data from the Crystallography Open Database #5910129
What is it?
Perhaps more popularly associated with the crime underworlds of Agatha Christie or clandestine operations in a war torn Europe, potassium cyanide is probably one of the most well-known and potent poisons. Very similar in appearance to regular white sugar, only 200mg would be enough for a lethal dose, so you wouldn’t want to get the two mixed up!
The crystal structure of potassium cyanide is very similar to that of sodium chloride (rock salt), forming a cubic lattice. The diatomic cyanide ions (CN-) spin so rapidly that their time averaged shape appears spherical, with differentiation between the two atoms only possible at low temperatures and high pressures.
Where did the structure come from?
Potassium cyanide has been the subject of numerous structural studies, but the first has been attributed to Richard M. Bozarth J. Am. Chem. Soc. 1922 44, 2, 317-323 and so is one of the earliest crystal studies!
Related articles
|
A bit of a mouthful – sodium vanadium fluorophosphates: Na3V2O2x(PO4)2F3-2x |
Kalgoorlie where the streets are paved with gold – Calaverite |
Porosity on your Plate – An Edible Metal-Organic Framework |