Inorganic structures
Posted on 26/02/2014

James Hester
Purple standards – Lanthanum hexaboride
What does it look like?
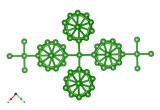
Four unit cells of LaB6, with atoms drawn according to their atomic radius. Created with DRAWxtl and Povray
What is it?
Lanthanum hexaboride (LaB6) is widely used as a high-performance source of electrons for electron guns in electron microscopes and other instruments. However, LaB6 is most familiar to crystallographers in its role as a beautiful purple standard material, for which it is well suited due to its stability and high crystallinity.
So how is it put together? A quick spot of X-ray or neutron crystallography will reveal that LaB6 is built out of a simple lattice of bulky lanthanum atoms, in the cavities of which nestle boron octahedra. Perhaps unusually, there are no atoms at the centre of these octahedra.
This simple structure proves to be more robust than most others in the natural world, with a melting point above 2200°C. Compare this to the 920°C melting point of pure lanthanum. So how does the addition of some tiny B atoms keep these La atoms from melting at high temperatures?
Well, the above description of B octahedra within a La lattice turns out [1],[2] to be the wrong way round: the B octahedra are in fact bonded very strongly to one another, forming a framework to which the La atoms are only weakly bonded, with very little La-La interaction as well.
It has been suggested that this is what makes LaB6 such a good material for electron guns: as the electrons are emitted and a surface La atom evaporates, a new La atom is easily able to migrate to the surface through the B framework as it is only very gently held in position.
[1]: http://dx.doi.org/10.1103/PhysRevB.72.235101
[2]: http://dx.doi.org/10.1063/1.2903150
Where did the structure come from?
The structure of Lanthanum hexaboride is #1000057 in the Crystallography Open Database.
Related articles
|
February's Birthstone – Amethyst |
A long chain crystal – Silver behenate |
Boron – an element to watch for the future |