Boron and carbon – better together?
What does it look like?
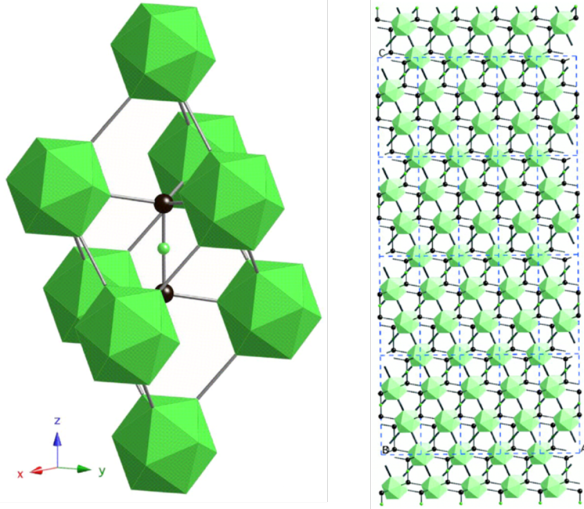
The ideal arrangement of Boron Carbide, layers of green boron clusters with the black carbon atoms between them.
What is it?
Boron carbide is a material that was discovered by accident in the 19th Century, and is (aside from boron nitride and diamond) one of the hardest materials known. Being cheaper to produce means that it is used for armoring tanks and in bullet-proof vests.
Unlike the super hard materials diamond and boron nitride, its crystal structure is actually quite complicated. It's known to have a roughly 4:1 ratio of boron to carbon atoms, but closer examination revealed that in practice there is always less carbon than expected in the structure. This deficiency of carbon atoms leads to some complexities in the crystal structure, which have been debated since the 1940s. Current thinking is that the structure is actually made up of a mixture of clusters of boron, some associated with more carbon and some with less.
Where did the structure come from?
We've pictured the 'ideal' arrangement of Boron carbide, but you can get an example of how complex the determination of this structure is from entry #2235962 in the Crystallography Open Database.