Not going anywhere – low expansion alloys
What does it look like?
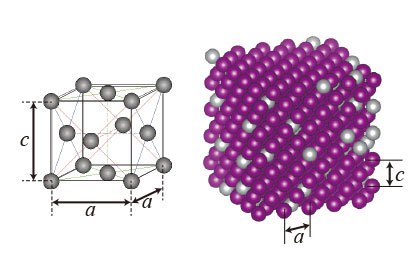
Details of the crystal structure of a low thermal expansion alloy (Manganese Nickel) investigated by Yokoyama et al. at the National Institute for Materials Science in Japan http://www.ims.ac.jp/english/topics/2012/130206.html. The purple atoms are the manganese and the silver atoms are nickel.
What is it?
One of the most fundamental things we can observe is that when we heat a material up it expands. This is a consequence of the heat transferring energy to the atoms within the material, and them moving about a bit more.
Metals often expand quite a bit, more than glass for instance (handy way to get a lid off a jar when it's stuck is to run the lid under a hot tap – it will expand more than the glass of the jar and should pop off a bit easier!). But there is a family of metal alloys that this wouldn't work for: low-expansion alloys.
The most famous of these is INVAR, which is an Iron Nickel alloy. The crystal structure is a simple face centred cubic structure, the left image in the picture, like gold for instance. INVAR is usually about 64 % iron, and 36 % nickel with these atoms existing in a solid solution - they are mixed up through the structure. The combination of these two elements in this ratio give a thermal expansion coefficient which is tiny, around 0.5 parts per million for every 1°C in heat increase. It's still not totally understood why this effect occurs.
There are actually a range of alloys in this family that have thermal expansion coefficient tuned to different materials. For instance, Kovar has the same expansion rate as glass so was used widely in the early electronics industry.
Where did these structures come from?
The discovery of low expansion alloys occurred around the 1890s and was deemed so important that the discoverer Guillame received the Nobel prize the year before Einstein! Being able to build a scientific instrument (particularly chronometers) that are inherently unaffected by temperature led to huge improvements in what could be done.