LixCoO2 – The Breakout Battery Hit
What does it look like?
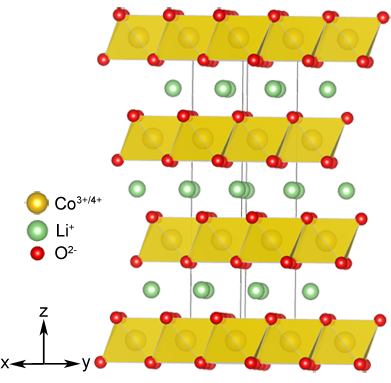
Image generated by the VESTA (Visualisation for Electronic and STructural analysis) software http://jp-minerals.org/vesta/en/
What is it?
LixCoO2 was first investigated for its magnetic properties by Sestrich et al.[1] and then identified as a lithium intercalation electrode in 1980 by Goodenough et al.[2] In 1991 Sony introduced rechargeable lithium ion batteries containing LixCoO2 and carbon black as the positive and negative electrodes respectively. This marked the beginning of the rapid expansion of lithium ion batteries as a power source for portable electronic devices.[3]
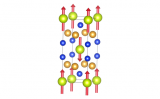
LixCoO2 adopts an "α‑NaFeO2" distorted rock-salt type superstructure. Within the close-packed anion array the Li and Co cations occupy the octahedral interstitial sites arranged such that the Li and Co are ordered into alternating layers along the [111] crystallographic direction. The CoO6 and LiO6 octahedra are edge sharing within and between planes. The image of the structure highlights the planes within which lithium can migrate. It is this layered structure which gives rise to many of the structural properties exhibited by LixCoO2. For instance the total amount of energy which can be stored is limited by the fact that battery cycling can only be performed within the range 0.5 ≤ x ≤ 1. If more lithium is removed repulsions between the CoO6 layers results in structural rearrangement. This issue has been overcome somewhat in new materials derived from LixCoO2 containing nickel. Due to the similar size of Ni2+ and Li+ anti-site disorder occurs between the lithium and nickel positions. That is, some lithium sits within the transition metal layer and vice versa for the nickel. The nickel cations within the lithium layer effectively act as "pillars" preventing layer collapse; however, too much anti-site disorder can inhibit lithium mobility.
Despite the challenges encountered for the LixCoO2 positive electrode material, it represents a significant advance in rechargeable lithium ion battery materials. Most significantly, it was the first positive electrode which acted as the lithium "source" in the battery. This allowed the highly reactive lithium metal, originally used as the negative electrode, to be replaced by carbon black. Every subsequent positive electrode material which has seen commercial success has exhibited this same property. Incidentally, the positive electrode being the lithium source is the reason why most portable electrode devices must be charged when first purchased.
Where did the structure come from?
Details for this structure can be found in entry #4505482 of the Crystallography Open Database.
[1] W.D. Johnston, R.R. Heikes, D. Sestrich, J.Phys. Chem. Solids 7 (1958) 1-13.
[2] K. Mizushima, P.C. Jones, P.J. Wiseman, J.B. Goodenough, Mat. Res. Bull. 15 (1980) 783-789.
[3] K. Ozawa, Solid State Ionics 69 (1994) 212-221.