Elements
Posted on 22/02/2014

Helen Maynard-Casely
The chemical bond is stronger than we could have imagined
There are at present a number of 'grand questions' in physics, from the search for the Higgs boson to the unbalance of matter and anti-matter in the observable universe; but the one that most interests me is the search for metallic hydrogen.

Why? It's the planetary implications. The planet Jupiter is very big and in the centre of it are extremes of conditions in terms of temperature and pressures that we cannot re-create here on Earth (yet). We've got a pretty good idea that the centre of Jupiter is going to be mostly hydrogen, as that is what most of the solar system is made of anyway. We know from gravity measurements that the centre of the planet is very dense. Jupiter also has a really interesting magnetic field. It's massive and if we could see its whole effect, it would be bigger than the Sun in our sky.
So we know something strange is going on in the centre of Jupiter (and indeed all the other gas giants). And we can even observe the effect of this strangeness in the massive magnetic field. Is this all because at massive pressures hydrogen, which we are all more familiar with as a gas, becomes a metal?
The theory goes that under extremely compressed conditions the hydrogen molecule breaks down, the electrons that orbit its nucleus will 'delocalise', and the material will become a metal. The first of these calculations, in 1935, predicted that this would occur at 250,000 atmospheres of pressure (equivalent to about 10 fully grown African elephants on one stiletto heel) but some of the first high-pressure experiments to get to those pressures soon found that this was not the case. Metallic hydrogen remained elusive.
In the last couple of months there have been two major finding reported in the search for metallic hydrogen. The first was from a group in Edinburgh, UK, who collected data from hydrogen at 3.15 million atmospheres. Using spectroscopy they found a distinct change in the behaviour of the material, indicating that the atoms within had changed their arrangement.
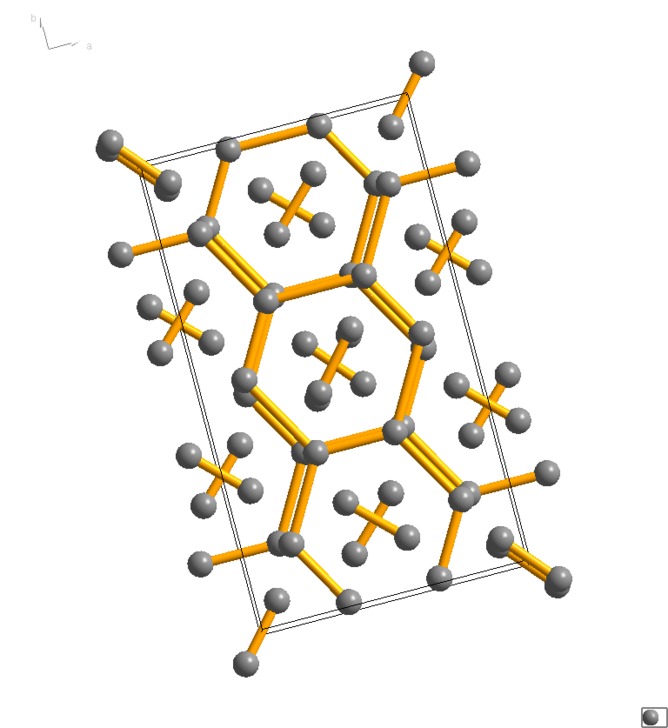
The possible structure of hydrogen at 3.15 million atmospheres.
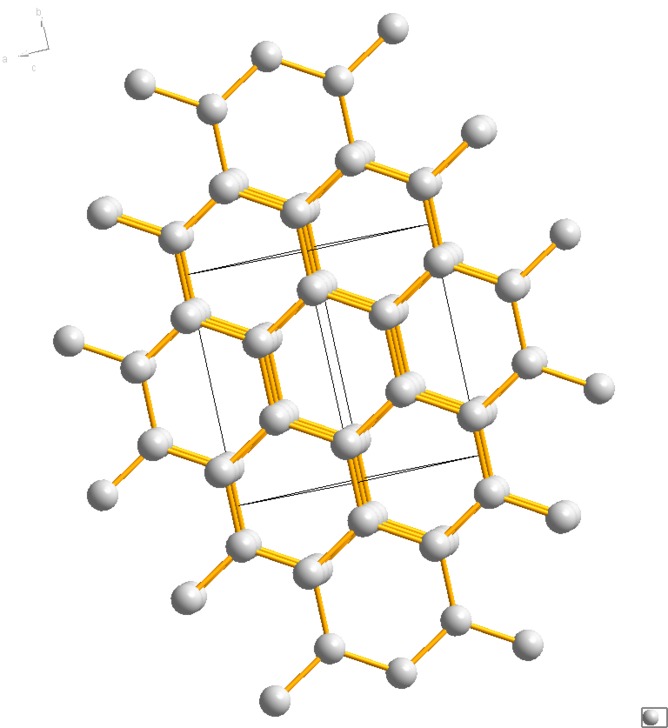
The structure of graphite
Looking to theory they noted the similarity of their measurements to those predicted by a graphite-like structure for solid hydrogen.
Then a group in Washington DC, USA, reported that they believe the hydrogen molecule is still intact and not metallic at 3.6 million atmopheres. They believe even at these pressures at least part of the structure is still made up of the H2 molecules that make up the hydrogen gas we are more used to. So now experimenters are at an impasse: it would seem the higher they go in pressure the more the hydrogen impresses them with the strength of its bond.
It all begins to beg the question what if hydrogen doesn't become metallic? Do the theoretical results need to be revised? Is there another way to describe Jupiter's magnetic field?
Hydrogen has long been used as a model system, because of its simplicity containing only one proton and one electron. The higher in pressure scientists observe hydrogen, the more complicated it would seem to become. So if even the simplest of things can be so perplexing, the mind boggles about what anything else in the universe will do!
![]()
This article was originally published on The Conversation.
Read the original article.
Related articles
|
The most recently discovered ice structure – Ice XV |
What is our planet made out of? (3) Wadsleyite |
Superhard and superstrong – cubic Boron Nitride |