Elements
Posted on 19/01/2014

Helen Maynard-Casely
Graphite – the makings of the future?
What does it look like?
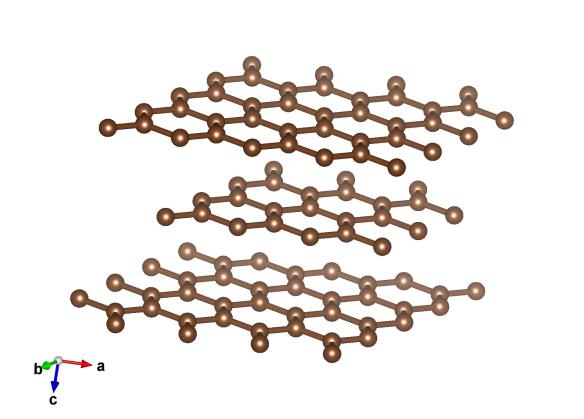
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
It’s what our ancestors (and some of us still) used to write with, and is only composed of one element carbon, but a feature of the structure of graphite could be shaping all of our futures. It's the more common form of carbon, and is made up of hexagonal sheets. It is often classed a 'semi metal' as large lumps of it are quite metallic-looking and, more critically, it can conduct electricity along the layers.
The sheets in graphite are only loosely connected together, which means that they can be prised apart. Perhaps the most successful pair to do this was Andre Geim and Kostya Novoselov, who used sticky tape to pull apart the layers of graphite. What they created doing this was 'Graphene', and delved into a new and exciting world of two-dimensional materials. For this work they won the 2010 Nobel Prize in physics, which honored their 'door opening' of a brand new world of strange and exotic properties that other scientists could explore. We’re yet to see everyday applications of graphene, but they are coming.
Where did the structure come from?
The structure of graphite was pondered for many years, with some incorrent structures published. The hexagonal structure was revealed by Lipson and Stokes in 1942, it is structure #120018 in the Crystallography Open Database.
Related articles
|
Not going anywhere – low expansion alloys |
Madam Curie – one of her elements: Curium |
Myoglobin: (Don't) hold your breath! |