Alpha plutonium – a rebel element
What does it look like?
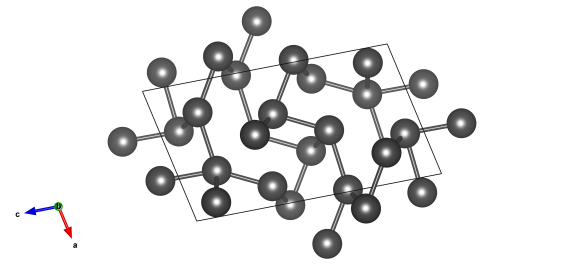
The crystal structure of alpha plutonium. Image generated by the VESTA (Visualisation for Electronic and STructural analysis) software http://jp-minerals.org/vesta/en/.
What is it?
Over the year we've introduced the crystal structure of a number of elements. Most of them take up very simple crystal structures, those that you can also form by pouring ping pong balls into a box. Atoms of materials like gold, krypton and even curium pack like this.
We've also introduced elements that don't conform to the 'simple crystal structure' rules at room temperature – the elemental rebels so to speak. These are the elements where the atoms are awkward – they need to be *that* bit further away from each other.
The latest of these 'rebel' elements is plutonium. This element actually has seven allotropes, with a face centred cubic phase (known as the delta-phase) stable at just above room temperature, 310 K. Below this it forms a crystal structure known as its alpha-phase, which is monoclinic, a low-symmetry structure that has low thermal conductivity and is quite brittle. Not great for use in reactors.
We've actually learnt how to tame the plutonium crystal structure somewhat. Alloy plutonium with a little bit of gallium, aluminium, americium, scandium or cerium and it forms into a gold-like face-centred cubic structure that isn't brittle and conducts heat much better.
Where did the structure come from?
The structure of alpha plutonium was discovered by Zachariasen and Ellinger 1963. It's #9008587 in the Crystallography Open Database.