Biological structures
Posted on 19/02/2014

Julia Archbold
Breaking down the drink – Alcohol Dehydrogenase
Today’s post is from Julia Archbold – she explains her inspiration: 'In order to give my poor liver a rest, I’ve decided to try "FebFast" this year. 28 days free from alcohol. I mean that's no wine for a whole month! I’m guessing my alcohol dehydrogenase enzyme is wondering what to do with itself at the moment, so I thought I’d give it a little bit of love and attention and tell you all about it.'
What does it look like?
There are many forms of alcohol dehydrogenase (ADH) in the body, with researchers identifying over 10 different subtypes. All ADH enzymes exist as a dimer, composed of two different subunits. Here is the structure of human liver beta3 beta3 alcohol dehydrogenase enzyme (subunit 1 coloured light pink, subunit 2 coloured hot pink). Each dimer contains two zinc ions (aqua dots), one of which is critical for its activity.
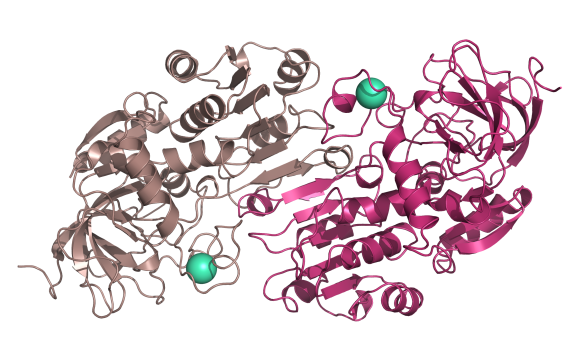
Image generated by Pymol (http://www.pymol.org/)
What is it?
Alcohol dehydrogenase is the enzyme responsible for converting alcohol to acetylaldehyde. Humans have evolved ADH to breakdown alcohol as alcohol (ethanol) is found everywhere in nature, from fruits to the yeast in our bread.
The different forms of the ADH enzymes all perform the same chemical reaction, but some work more efficiently than others. This is why alcohol has differing effects on different people. Indeed, variations in ADH enzymes have been linked with alcoholism.
Women, on average, have less ADH in their stomach, which is why woman are advised to drink less than males. Older males produce less ADH, explaining why your grandpa gets drunk more quickly than your brother. However, frequent heavy drinkers produce more ADH than others, meaning they can metabolise alcohol more efficiently than others.
Where did the structure come from?
The first structure of the first mammalian liver alcohol dehydrogenase came from the horse in 1973. Many human homologues have been solved since, including the human beta1beta1 ADH in 1991 and the human beta3beta3 ADH structure was published in 1996.
Related articles
|
HLA Molecules – How the body detects infected cells |
Fighting the Flu – The structure of Neuraminidase |
Cold as Ice… with 'Maxi' sacrifice |