Biological structures
Posted on 24/03/2014

Róisín McMahon
Myoglobin: (Don't) hold your breath!
A short break from our rainbow coloured crystal structures, as today would be John Kendrew's birthday. John Kendrew received a Nobel Prize with Max Perutz for being the first to determine a protein structure – and it was this one!
What does it look like?
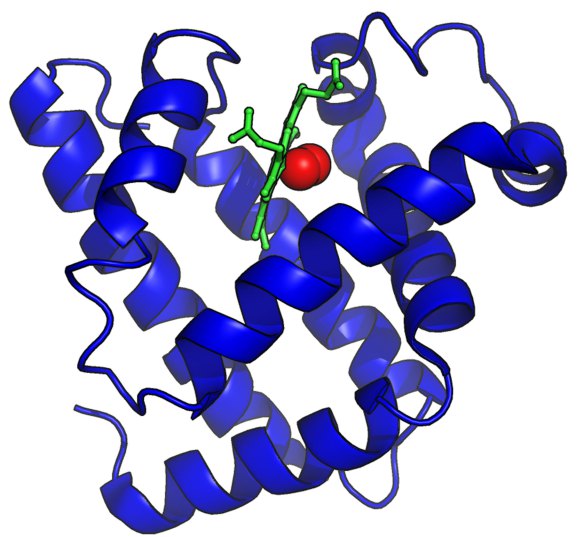
Cartoon representation of oxymyoglobin isolated from the sperm whale (PDB ID 1MBO1). Myoglobin is shown in cartoon representation (blue) in complex with its iron haem group (green) and molecular oxygen (red spheres.) The image was generated using the molecular graphics software PyMOL.
What is it?
Ever wonder why seals and whales can dive for up to an hour on a single breath, but you can’t make to the other end of the swimming pool? The answer, in part, is myoglobin.
Myoglobin is a specialised oxygen carrier protein, similar to the better-known haemoglobin. Whilst haemoglobin circulates in the blood and facilitates oxygen transport, myoglobin is present in the skeletal muscle (i.e. those muscles that allow you to move) where it enables oxygen storage.
Oxygen transport in the body relies on the fact that oxygen binding proteins pick up and hold onto oxygen when they are in an environment where oxygen concentration is high (e.g. the lungs), and later give up that oxygen when they are in an environment where oxygen concentration is low (e.g. the brain, exercising muscles) thus delivering it to where it is needed. Haemoglobin and myoglobin both deliver oxygen, but they differ in how low the oxygen concentration (more precisely the oxygen partial pressure) of the local environment has to be before they will release their precious cargo. Haemoglobin can give up oxygen quite readily, but myoglobin only releases oxygen when the local concentration of oxygen is much lower; this difference effectively allows myoglobin to store oxygen reserves in the muscles ready for future periods of activity.
Seals, whales and other deep diving sea mammals have a much higher concentration of myoglobin in their muscles than humans. This is crucial for their ability to take long underwater dives. Typically when proteins are very highly concentrated in one place, they start to clump together, preventing them from functioning properly. Deep diving sea mammals however have evolved variants of myoglobin that have a very highly charged surface that effectively makes them less "sticky" and allows them to remain at high concentrations without a loss of function. 2
Where did the structure come from?
The crystal structure of myoglobin was determined by John Kendrew and colleagues using protein that they isolated from sperm whales. 3,4 It was the first protein structure to be solved by protein crystallography. Kendrew subsequently shared the 1962 Nobel Prize for Chemistry with Max Perutz "for their studies of the structures of globular proteins".
- S. Phillips. JMB, 142:531-554 (1980)
- S. Mirceta et al., Science 340, 1234192 1-8 (2013)
- J. Kendrew et al., Nature 181, 662-666 (1958)
- J. Kendrew et al., Nature 185, 422-427 (1960)
Related articles
|
P-glycoprotein: It's as simple as ABC |
Not going anywhere – low expansion alloys |
DNA: Life's blueprint |