An explosive discovery – Einsteinium sesquioxide
What does it look like?
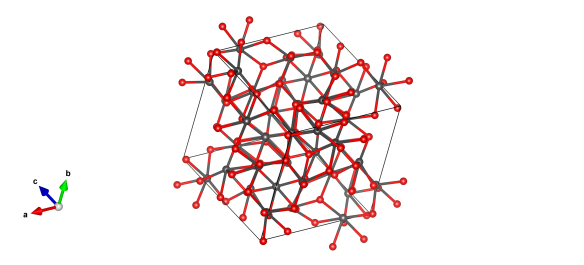
The structure of Einsteinium sesquioxide – the red atoms are oxygen and the grey atoms represent Einsteinium.
What is it?
So we wouldn’t really be a 'science' blog if we didn't mention probably the most famous scientist of all at some point. … Today marks 99 years since Albert Einstein published his paper 'Does the Inertia of a Body Depend Upon Its Energy Content?', the insight that lead to the oft quoted E=mc2 equation.
In recognition of the great steps Einstein made to fundamental science, he was afforded the rare honour of having an element named after him. Its discovery was perhaps a little dramatic. Einsteinium was first noticed as a remnant of the first hydrogen-bomb in 1952. The most common isotope of Einsteinium (Es-253) can be produced in some of the more powerful reactors in the world from the decay of californium-253.
It is a soft, silvery metal that is seen to oxidise to a sesquioxide, which is the structure we've featured today. In a further interesting note, sesquioxidizing, meaning the creation of a sesquioxide, is the highest scoring word that would fit on a Scrabble board.
Where did the structure come from?
The crystal structure of Einsteinium sesquioxide was determined by Haire and Baybarz in 1973. There's not an entry for it in the Crystallography Open Database - instead, we took the entry for Gadolinium sesquioxide (#1010338) and altered the lattice parameters and changed the Gd atoms for Es ones.