Feeding the world – Ammonia
What does it look like?
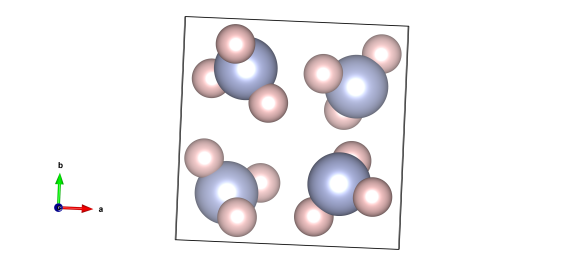
The crystal structure of frozen ammonia. The light blue atoms are nitrogen and the pink ones are hydrogen. Image generated by the VESTA (Visualisation for Electronic and STructural Analysis) software http://jp-minerals.org/vesta/en/
What is it?
Though it's a rather noxious gas at room temperature, ammonia will freeze at -77 °C. At this point it forms a rather simple cubic structure. Ammonia is one of the main ingredients for fertilisers, without which it's estimated that we couldn't have fed over a third of the world's population.
Often referred to as the most important discovery of the 20th Century, but probably very few people have heard of the Harber–Bosch process. This process produces ammonia by heating and pressurising hydrogen and nitrogen gas in the presence of some iron, and is vital for feeding the world's population.
Despite the success of the Haber-Bosch process, it is incredibly energy intensive, taking up over a third of the world's energy. On top of this the production of the pure hydrogen has carbon dioxide as a by-product. In the last few days, scientists from George Washington University have announced that they may have found a way to make ammonia from water and air (instead of pure hydrogen and nitrogen gas). It's hoped that this less energy-intensive process could revolutionise the way we produce ammonia.
Where did the structure come from?
As we've mentioned a couple of times before, finding hydrogen-atom positions in structures like this is quite tricky - this sort of structure couldn't be solved with the more conventional X-ray diffraction. Instead the authors had to use a beam of neutrons, and studied deuterated (hydrogen with a neutron added) ammonia. This scatters really well, and allowed the authors Hewat and Reikel to pinpoint the position of these atoms.